The bent bond / antiperiplanar hypothesis and the thermal rearrangement of cyclopropyl halides and tosylates
The mechanism of solvolysis of cyclopropyl halides and tosylates yielding allylic products is deduced at the molecular level through the Bent Bond/Antiperiplanar Hypothesis (BBAH). The cleavage of the cyclopropane bond occurs in a concerted manner with the departure of the leaving group. This rearrangement takes place when the pyramidal diradical intermediate derived from the structure of the cyclopropane τ bond is antiperiplanar to the halide or sulfonate leaving group.
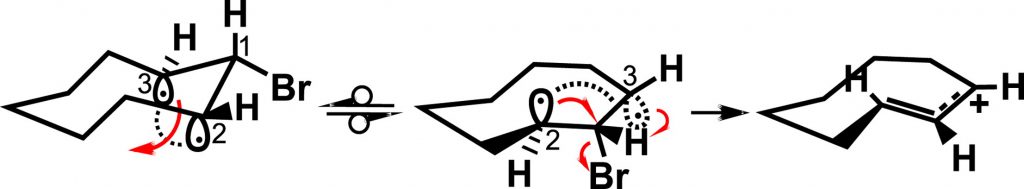
BBAH #12: P. Deslongchamps, The bent bond / antiperiplanar hypothesis and the thermal rearrangement of cyclopropyl halides and tosylates, Tetrahedron, 2020, 76, 131416-131420.
https://doi.org/10.1016/j.tet.2020.131416
Be the first to comment