The Bent Bond/Antiperiplanar Hypothesis (aka BBAH) is a conceptually novel orbital model for rationalizing the conformation and stereochemical reactivity of organic molecules containing various types of unsaturation. The BBAH is based on the Slater-Pauling bent bond model (tau bond, 𝜏 bond) and takes into consideration the antiperiplanar hypothesis, a stereoelectronic concept to describe electronic delocalization in unsaturated systems.
| Table of Contents |
| Bent bonds Antiperiplanar Hypothesis Anomeric effect Walden Inversion Next steps Publications |
Bents bonds:
The concept of bent bonds is not new. Linus Pauling and John Slater had described in the early 1930’s how double bonds could be represented by two different orbital models. In the Hückel model, the double bond consists of a σ and and a π bond (Fig. 1) whereas in the Slater-Pauling model, two bent bonds (𝜏 bonds) are formed between two largely tetrahedral carbons (Fig. 2).
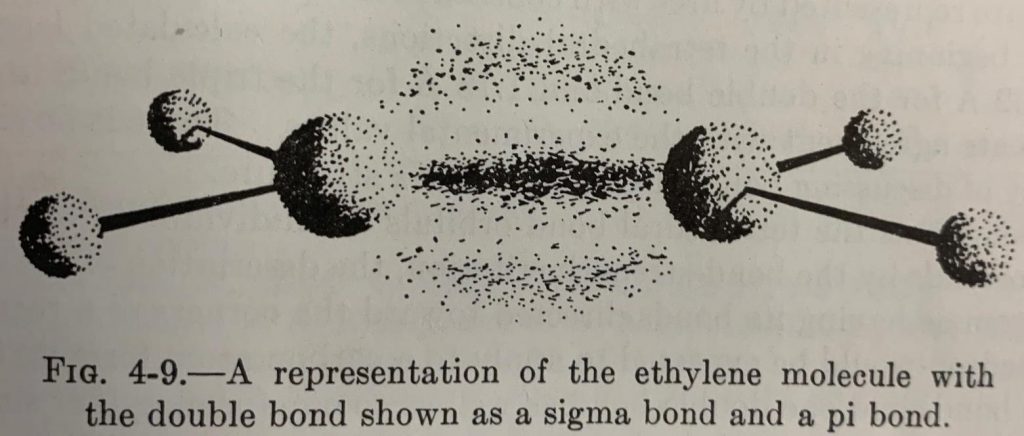
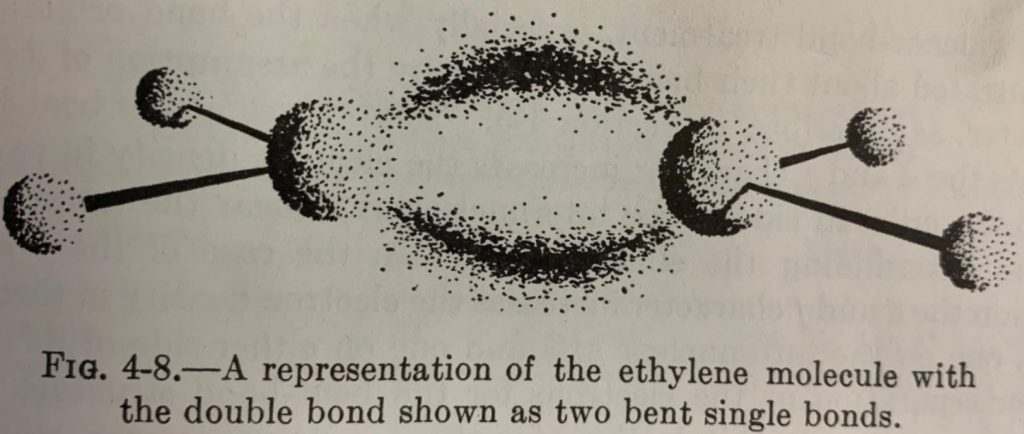
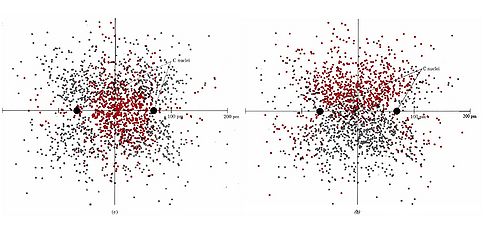
The two models are quantitatively equivalent from the standpoint of molecular orbital (MO) theory, each being interconvertible via unitary transformation of the basic atomic orbital functions. In simpler terms, linear combinations of the σ/π orbitals of the Hückel model produces the 𝜏 orbitals of the Slater-Pauling model with the same overall electron density (Fig. 3).
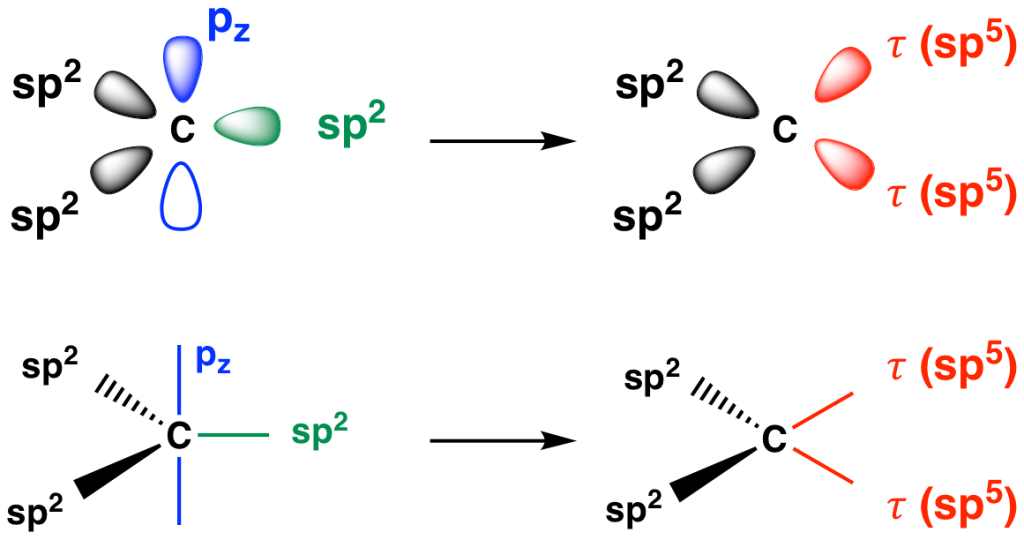
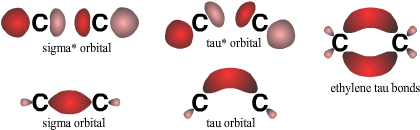
Indeed, linear combination of one of the hybrid orbitals of a sp2-hybridized carbon with its remaining pz orbital produces two sp5 hybridized 𝜏 orbitals, each with 1/6 s character and 5/6 p character (Fig. 4). The 𝜏 orbitals have the proper geometry for forming two bent bonds with other atoms. The bent bond model accounts for the observed bond lengths and angles in a wide range of C=C, C=X, and N=O compounds. Although the Hückel model has gained wide adoption, the Slater-Pauling model remains valid to this day.
The resulting 𝜏 bond orbitals in ethylene can be conceptualized as curved σ and σ* orbitals (Fig. 5). The antibonding 𝜏* orbitals come into play when considering the antiperiplanar hypothesis.
Antiperiplanar hypothesis:
The antiperiplanar hypothesis is a well-known stereoelectronic concept. It governs a wide range of organic structures and their reactivities in which a pair of electrons, either bonded or non-bonded, prefers to be antiperiplanar to some other molecular group, typically electron-withdrawing in nature.
Anomeric effect
The anomeric effect is a stereoelectronic phenomenon affecting both conformation and reactivity in carbohydrates and, in general, acetal-related functional groups. It is a fundamental examplar of the antiperiplanar hypothesis at work. In these systems, the presence of a lone pair, if antiperiplanar to a polar bond, contributes to their conformational stability and reactivity.
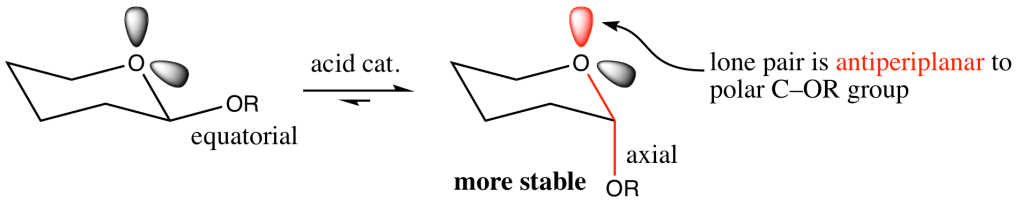
For example, the mutarotation of glycosides, in which the anomeric group can isomerize under acidic conditions, favours the formation of the axial epimer despite its greater steric hindrance compared to its equatorial counterpart (Fig. 6). Thus, a stereoelectronic effect counteracts the steric preference for groups on cyclohexane-like frameworks to be equatorial. In the axial epimer, the axial oxygen lone pair is antiperiplanar to the polar OR bond. This allows for delocalization of the lone pair into the antibonding σ* orbital of the exocyclic C–OR bond, referred to as a n → σ* interaction, a stabilizing form of negative hyperconjugation (Fig. 7).
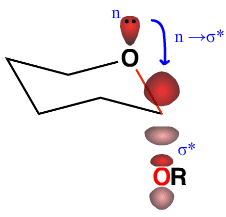
The anomeric effect can be viewed as a stabilizing hyperconjugation between a lone pair (n) delocalizing into the vacant σ* orbital of an adjacent polar bond (C–OR). The required orbital overlap is optimal if the lone pair is antiperiplanar to the polar bond.
What does this have to do with bent bonds? In valence bond theory, the anomeric effect can be viewed as a bond/no-bond resonance between a single bond structure and a bent bond form. (Fig. 8). The same analogy can be used to describe C=C bent bonds as resonance structures.
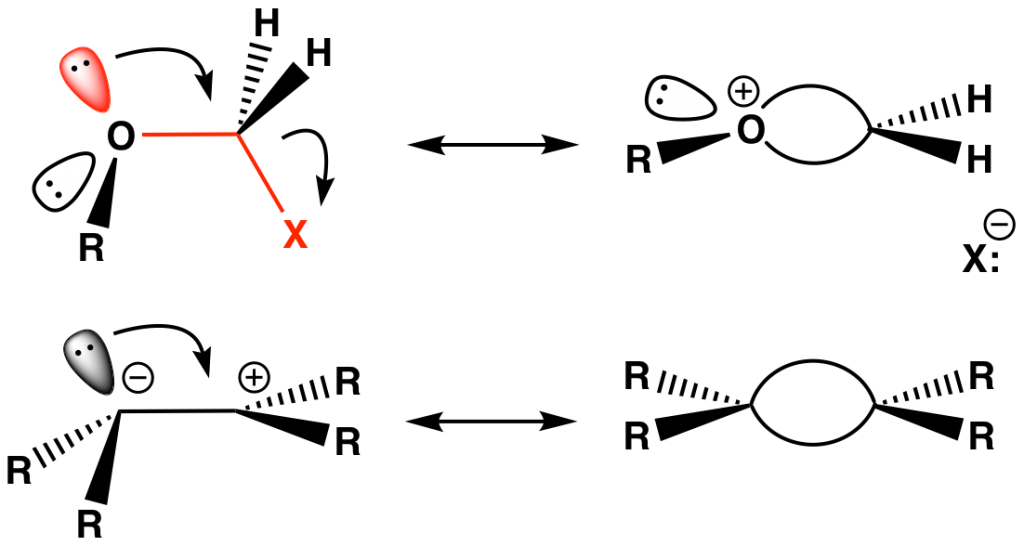
The antiperiplanarity concept can be generalized as a stabilizing hyperconjugative effect between a lone pair or a bond that is antiperiplanar to a polar bond. For example, elimination reactions can be viewed as the removal of a β-hydrogen by base only if it is antiperiplanar to the leaving group X (Fig. 9). The incipient lone pair produced during hydrogen abstraction can delocalize into the vacant C–X σ* orbital as the elimination takes place. Increasing the electron-withdrawing nature of the leaving group X enlarges the backside lobe of the C–X σ* orbital, improving overlap with the incoming pair of electrons released from the scissile C–H bond.
Walden inversion
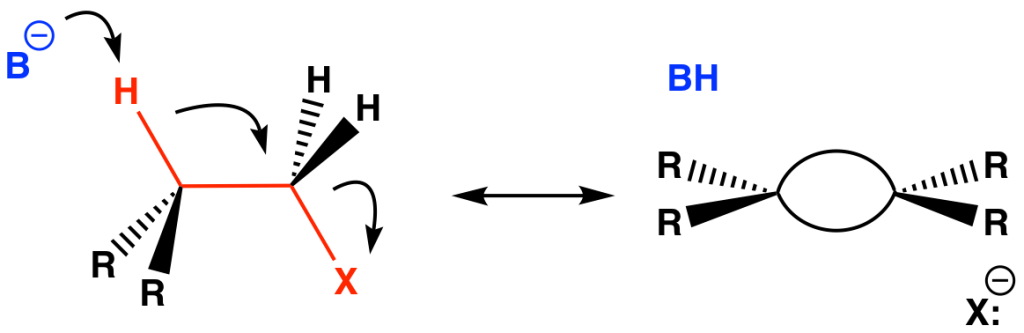
The Walden inversion refers to the inversion of configuration of a tetrahedral atom, usually carbon, as it undergoes nucleophilic substitution. It is a key concept behind the SN2 mechanism whereby the incoming nucleophile does a “backside attack” of the electrophile such that it is 180° from the departing leaving group. Apart from steric considerations, the trajectory has a stereoelectronic component in which the nucleophile lone pair overlaps with the backside lobe of the C–X σ* orbital, a n→σ* interaction (Fig. 10).
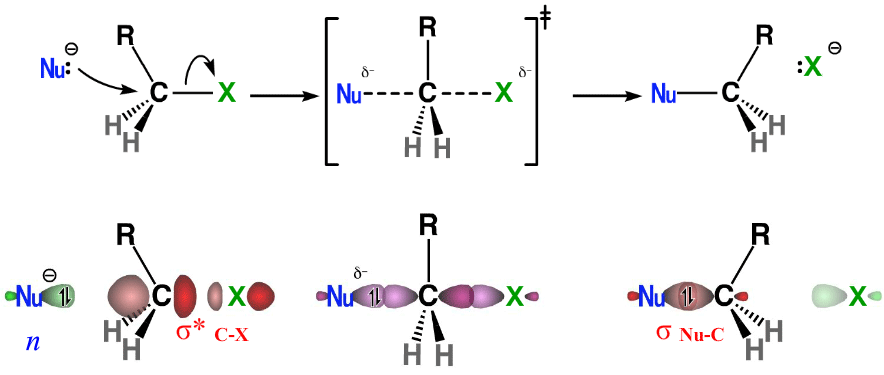
Because a bent bond confers tetrahedral character to its two bonded atoms, principles that apply to saturated carbon can also apply to unsaturated systems. For example, the attack of a nucleophile to a C=C bond (or more commonly a C=O bond) is viewed as a SN2-like displacement of a bent bond with Walden-like inversion at the electrophilic carbon (Fig. 11). The stereoelectronic requirement for backside attack dictates that only the bent bond that is anti to the incoming nucleophile undergoes opening. It also implies that the incipient lone pair will end up antiperiplanar to the bonded nucleophile, and that the immediate product is in a staggered conformation. The reverse of these reactions corresponds to an anti elimination process (Figs. 8 and 9).
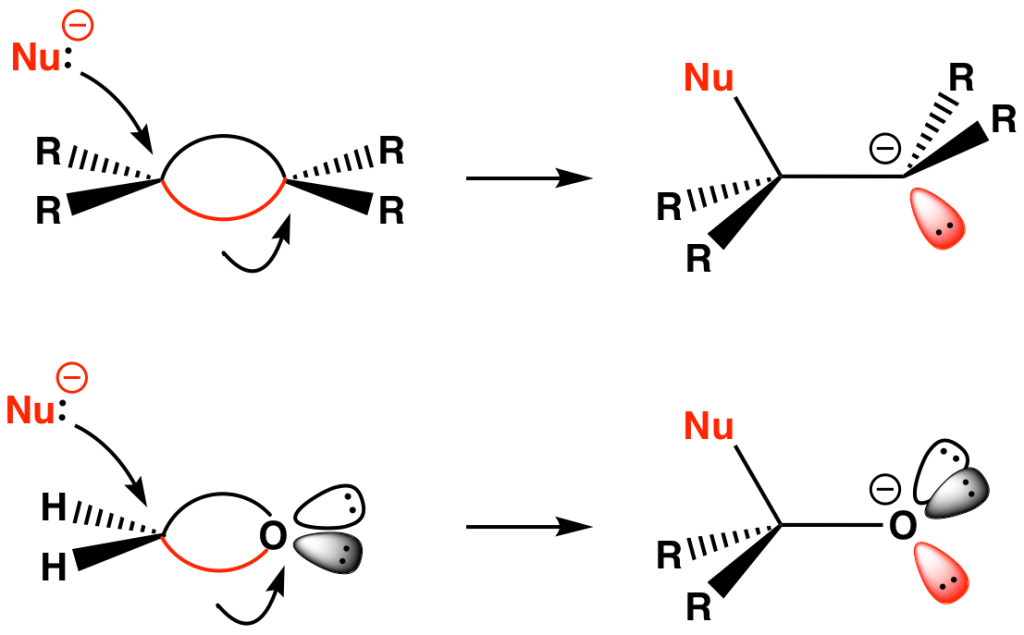
From a localized orbital standpoint, these addition reactions can be viewed as bent bond equivalents of SN2 reactions, corresponding to n→τ* interactions (Fig. 12). The trajectory of nucleophilic attack remains consistent with that described by Burgi and Dunitz.

Next Steps
The stereoelectronic requirement for bent bonds to be displaced in SN2-like dictates the antiperiplanarity principle! This interdependence forms the basis of the bent-bond/antiperiplanar hypothesis (BBAH):
– Bent bonds form by intramolecular antiperiplanar closures (Figs. 6-9)
– Bent bonds are opened in SN2-like fashion, resulting in an antiperiplanar lone pair (Figs. 11-12).
Subsequent blog posts describe the various applications of the BBAH on the conformational properties of molecules and on an ever-widening scope of organic reaction classes.
Be the first to comment