Bent Bonds (τ) and the Antiperiplanar Hypothesis – the Chemistry of Cyclooctatetraene and other C8H8Isomers
The fascinating chemistry of cyclooctatraene, including its propensity for ring inversion, bond shifting, and valence isomerization, is analyzed by the BBAH.
L. T. Scott discovered the 1,2-swapping of carbon and hydrogen atoms which is known to take place on benzenoid aromatics (up to ∼ 1000 ° C range). For example, 13C-1-naphthalene is specifically converted to 13C-2-naphthalene, and there is evidence that this occurs through the formation of benzofulvene and a naphthalene− carbene intermediate. Application of the bent bond/antiperiplanar hypothesis leads to the postulate that higher in energy pyramidal singlet diradical intermediates can be used to propose a mechanism that rationalizes various atom rearrangements on benzenoid aromatics and related isomeric compounds.
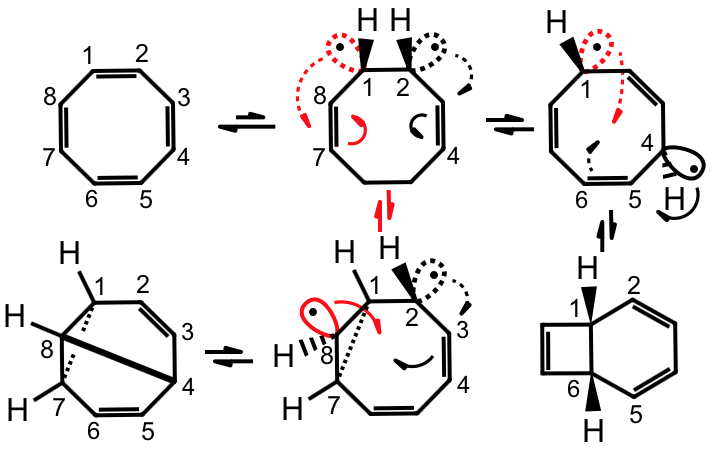
G. Deslongchamps, P. Deslongchamps, Bent Bonds (τ) and the Antiperiplanar Hypothesis – the Chemistry of Cyclooctatetraene and other C8H8Isomers. J. Org. Chem., 2018, 83, 5751-5755.
https://doi.org/10.1021/acs.joc.8b00809
Be the first to comment