Bent bond / antiperiplanar hypothesis and antiaromatic, aromatic and nonaromatic molecules
The stereochemical reactivities of monocyclic unsaturated compounds having 3–5 and 7–8 membered rings and of bicyclic pentalenes and heptalene can be rationalized through the bent bond / antiperiplanar hypothesis (BBAH) orbital model at the molecular level. This new orbital model considers unsaturations as Pauling τ bonds and that electron delocalization with adjacent pyramidal radical structures (derived from resonance structures) must strictly occur in antiperiplanar fashion. The BBAH shows that, contrary to aromatic (4n+2 Hückel) and nonaromatic molecules, antiaromatic molecules (4n Hückel) have adjacent double bonds that cannot conjugate through antiperiplanar electronic delocalization.
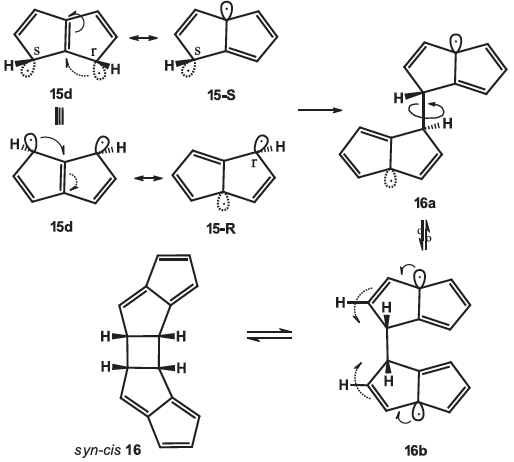
BBAH #15: P. Deslongchamps, Bent bond / antiperiplanar hypothesis and antiaromatic, aromatic and nonaromatic molecules, Tetrahedron, 2021, 89, 132161-132183.
https://doi.org/10.1016/j.tet.2021.132161
Be the first to comment