Applying the bent bond / antiperiplanar hypothesis to the stereoselective glycosylation of bicyclic furanosides
The glycosylation stereoselectivities for a series of bicyclic furanoside models have been carried out in the presence of weak nucleophiles. These results were analyzed through the bent bond / antiperiplanar hypothesis (BBAH) orbital model in order to test its validity. According to the BBAH, incoming nucleophiles displace one of the two bent bonds of bicyclic oxocarbenium ion intermediates in antiperiplanar fashion. The glycosylation stereoselectivity is then governed by displacement of the weaker bent bond as determined by the presence of electron-withdrawing or donating substituents at C2. Overall, the BBAH analysis expands Woerpel’s “inside/outside attack” glycosylation model by considering the stereoelectronic influence of neighbouring electron-withdrawing and donating groups on the nucleophilic addition to oxocarbenium ion intermediates
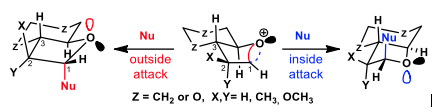
BBAH #9: J.-F. Parent, X. Bertrand, G. Deslongchamps, P. Deslongchamps, Applying the bent bond / antiperiplanar hypothesis to the stereoselective glycosylation of bicyclic furanosides. J. Org. Chem., 2020, 85, 758-773.
https://doi.org/10.1021/acs.joc.9b02791
Be the first to comment